| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
The ultimate example of delayed crazing: 90 years!
Glaze chemistry is the key to understanding it
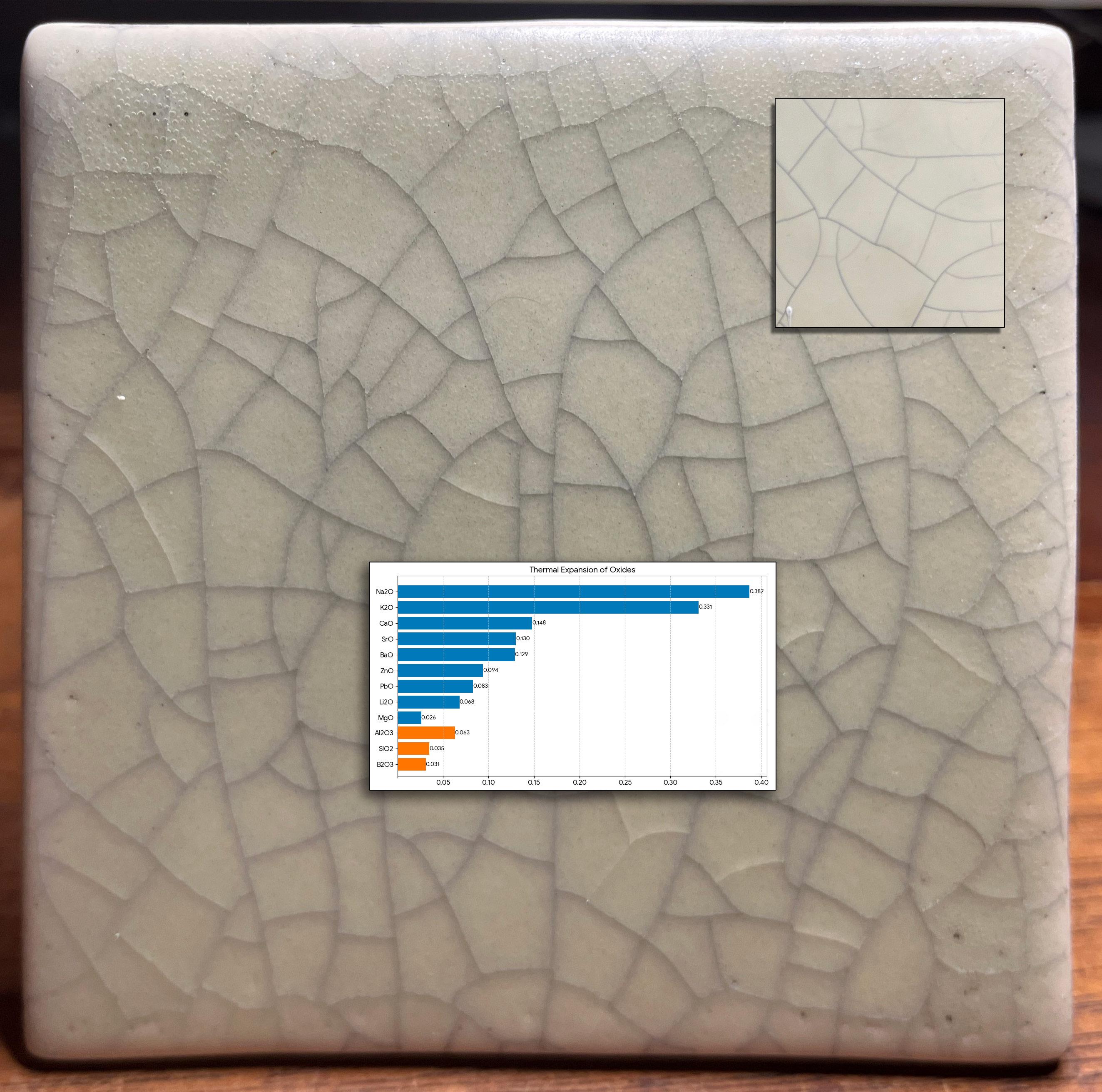
A restoration project faced a tile-matching challenge. At installation in a bathroom 90 years ago, the tiles were not crazed. But between then and now it happened (shown inset upper right). Now, a restoration specialist is tasked with duplicating the aged effect (one unsuccessful attempt is shown here). The shade, opacity, degree of matteness, bubble-free matrix and surface character of the original are all real challenges. Duplicating the crazing is even more difficult. Why? Matching "time-crazing" with a crackle glaze pattern will be temporary (it will craze much more after installation).
The reason why functional mattes seldom craze can be seen in the chemistry. This chart compares the thermal expansions of the oxides that combine to form the fired glaze matrix. ~80%+ of the makeup of almost all common base glazes (without colorants, opacifiers) is SiO2 and Al2O3 (orange bars). Mattes almost always need a low Si:Al ratio (e.g. below 6:1). The rest is fluxing oxides to melt them (the blue bars + B2O3). Here is the problem with making a crazing matte: Almost all crazing is caused by high levels of K2O and/or Na2O (the top two bars on the graph). But they produce high gloss (as can be seen in this test tile). The main matting fluxes and agents are MgO, CaO, SrO, BaO; they have a low COE (and don't craze glazes). Further, both zircon and tin oxide, the opacifiers needed, also have low thermal expansions!
Other possibilities of making crazed matte:
-A matte glaze can have a high SiO2:Al2O3 ratio and craze if it is very melt fluid (containing lots of KNaO) and cooled slowly so that micro-crystals cover the surface. The downside is unpleasantness to the touch.
-Glossy glazes can be matted by the addition of micron-fine alumina (e.g. 800 mesh, this is done in the tile industry).
-A low expansion body with no ball clay or silica (e.g. just kaolin and feldspar with enough bentonite to get the needed plasticity) will craze most glazes. Adding pyrophyllite will further lower its COE.
-Print the lines on the tile (using ceramic transfers) and use a translucent matte glaze (like G2934).
Related Pictures
Turning delayed crazing into immediate crazing

This picture has its own page with more detail, click here to see it.
This is a cone 04 clay (Plainsman Buffstone) with a transparent glaze (G1916Q which is 65% Frit 3195, 20% Frit 3110, 15% EPK). On coming out of the kiln, the glaze looked fine, crystal clear, no crazing. However, when heated to 300F and then immersed into ice water this happens. This is the IWCT test. At lower temperatures, where bodies are porous, water immediately penetrates the cracks and begins to waterlog the body below. Fixing the problem was easy: Substitute the low expansion Frit 3249 for high expansion Frit 3110.
Videos
Links
| Troubles |
Glaze Crazing
Ask the right questions to analyse the real cause of glaze crazing. Do not just treat the symptoms, the real cause is thermal expansion mismatch with the body. |
Got a Question?
Buy me a coffee and we can talk

https://backup.digitalfire.com, All Rights Reserved
Privacy Policy