| Monthly Tech-Tip | No tracking! No ads! | |
Oxide Formula
In ceramics, the chemistry of fired glazes is expressed as an oxide formula. There are direct links between the oxide chemistry and the fired physical properties.
Key phrases linking here: oxide chemistries, oxide formulas, oxide formula, oxide chemtry - Learn more
Details
Conceptually we consider fired ceramic glazes as being composed of 'oxides' (materials contribute these). Ten major oxides likely make up 99% of all base glazes (and materials we use). The oxide formula of a glaze "explains" many details about the way it fires (provided all the materials in the glaze dissolve in the melt). There is some redundancy with this term, sometimes people refer to "glaze formulas" when what they actually mean is "glaze recipes".
A formula expresses an oxide mix according to the relative numbers of molecule types (in contrast to a chemical analysis that compares them by weight). A formula is suited to analyzing and predicting properties of a fired glaze or glass because it gives us a picture of the molecular structure that is responsible for fired behavior. Since the kiln fires build these oxide molecules one-by-one into a structure, it follows that one will never fully 'understand' why a glaze fires the way it does without seeing its oxide formula.
Formulas are flexible. We can arbitrarily retotal one without affecting the relative numbers of oxide molecules. In fact, this retotaling of a formula is standard procedure to produce a Seger unity formula or a Mole% formula. With a formula, you need not worry whether there is 1 gram, 1 ton, or one billion molecules, only relative numbers matter. This is why it is allowable to express a formula showing molecule parts (e.g. 0.4 MgO; in reality this would not occur, but on paper a formula helps us compare relative numbers of oxide molecules in a ratio).
An example of a raw formula:
| Fluxes RO |
Intermediates R2O3 |
Glass Formers RO2 |
|||
| K2O CaO MgO ZnO |
0.5 1.3 0.2 0.1 |
Al2O3 | 0.9 | SiO2 | 9.0 |
Notice in the above that oxides are grouped into three columns: the bases, acids, and amphoterics (or simply as the RO, R2O3, and RO2 oxides; where "R" is the element combining with oxygen). Actually, the ratio of R to O is significant. The right column has the greatest oxygen component, the left has the least. Simplistically, we can view these three groups as the silica:alumina:fluxes system. This latter convention is not really correct because there are more glass builders than SiO2 , other intermediates besides Al2O3, and the RO's do more than just flux. Ancient potters referred to these three as the blood, flesh, and bones of a glaze (not a bad way to think of it).
Any formula has a formula weight, that is, the total calculated weight for that mix of molecules. Atomic weights are published in any ceramic text so it is easy to calculate the weight of each oxide. Calculating the weight of the whole is just a matter of simple addition and then increasing that weight to account to LOI.
Insight-live normally determines the unity of formulas it calculates automatically. If the RO oxides total is greater than R2O3/2 it is deemed flux unity. Otherwise it is R2O3 unity.
Related Information
Example of a whole rock chemical analysis lab report

This picture has its own page with more detail, click here to see it.
Powdered samples were sent to the lab. The numbers shown on this report are in percentage-by-weight. That means, for example, that 15.21% of the weight of the dry powder of Alberta Slip is Al2O3. Insight-live knows material chemistries in this way (whereas desktop Insight needs them as formulas). Some non-oxide elements are quantified as parts-per-million (these amounts are not normally high enough to take into account for traditional ceramic purposes). The LOI column shows how much mechanically and chemically bound water are gassed off during firing of the sample. The total is not exactly 100 because of inherent error in the method and compounds not included in the report.
Desktop INSIGHT showing formula and analysis side-by-side

This picture has its own page with more detail, click here to see it.
Desktop Insight was the first to enable users to compare two recipes and their formulas side-by-side and interactively update when recipe changes were made. It also enabled users to show formulas and analyses side-by-side.
Ceramic Oxide Periodic Table

Pretty well all common traditional ceramic base glazes are made from less than a dozen elements (plus oxygen). Go to the full picture of this table and click or tap each of the oxides to learn more (on its page at digitalfire.com). When materials melt, they decompose, sourcing these elements in oxide form. The kiln builds the glaze from them, it does not care what material sources what oxide (assuming, of course, that all materials do melt or dissolve completely into the melt to release those oxides). Each of these oxides contributes specific properties to the glass. So, you can look at a formula and make a good prediction of the properties of the fired glaze. And know what specific oxide to increase or decrease to move a property in a given direction (e.g. melting behavior, hardness, durability, thermal expansion, color, gloss, crystallization). And know about how they interact (affecting each other). This is powerful. A lot of ceramic materials are available, hundreds - that is complicated when individual materials source multiple oxides. Viewing a glaze as a simple unity formula of ceramic oxides is just simpler.
The Seger Unity Formula

This picture has its own page with more detail, click here to see it.
Ceramic material powders can be crystalline or amorphous (or a combination) and they can be natural or man-made and they can have very different particle physics. Ceramic materials are classic case studies of the sciences of solid state physics and solid state chemistry. Mineral sources of oxides impose their own melting patterns - when one is substituted for another, to supply an oxide, a different system with its own relative chemistry is entered. An extreme example of this is sourcing Al2O3 to a glaze using calcined alumina instead of kaolin. Although the chemical formula of the glaze may remain unchanged, the fired result would be completely different. This is because very little of the alumina would dissolve into the glaze melt. At the opposite extreme, a different frit could be used to supply a set of oxides (while maintaining the overall chemistry of the glaze) and the fired result would be much more chemically predictable. Why? Because they readily release their oxides to the melt.
A limit or target glaze formula. What does this mean?

This picture has its own page with more detail, click here to see it.
Recipes show us the materials in a the glaze powder (or slurry). Formulas enumerate the oxide molecules and their comparative quantities in the fired glass. Oxides construct the fired glass. The kiln de-constructs ceramic materials to get their oxides, discards the carbon, sulfur, etc. and builds the glass from the rest. There is a direct relationship between fired glaze properties (e.g. melting range, gloss, thermal expansion, hardness, durability, color response, etc) and its oxide formula. There are 8-10 oxides to know about (vs. hundreds of materials). From the formula-viewpoint materials are thus "sources-of-oxides". While there are other factors besides pure chemistry that determine how a glaze fires, none is as important. Insight-live can calculate and show the formula of a recipe, this enables comparing it side-by-side and with a target formula (or another recipe known to work as needed). Target formulas are opened using the advanced recipe search, choosing the limits batch and clicking/tapping the search button (search 'target recipe' in Insight-live help for more info).
Insight-Live comparing a glossy and matte cone 6 base glaze recipe
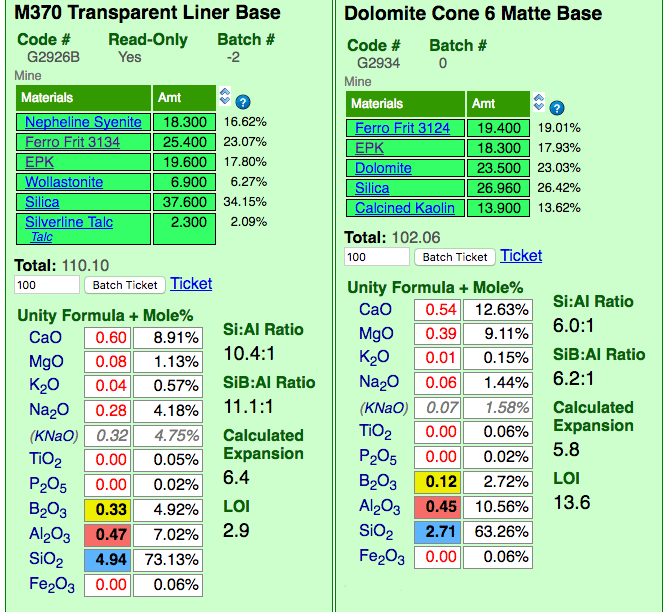
This picture has its own page with more detail, click here to see it.
Insight-live is calculating the unity formula and mole% formula for the two glazes. Notice how different the formula and mole% are for each (the former compares relative numbers of molecules, the latter their weights). The predominant oxides are very different. The calculation is accurate because all materials in the recipe are linked (clickable to view to the right). Notice the Si:Al Ratio: The matte is much lower. Notice the calculated thermal expansion: The matte is much lower because of its high levels of MgO (low expansion) and low levels of KNaO (high expansion). Notice the LOI: The matte is much higher because it contains significant dolomite.
Links
| Glossary |
Formula Weight
|
| Glossary |
Ceramic Oxide
In glaze chemistry, the oxide is the basic unit of formulas and analyses. Knowledge of what materials supply an oxide and of how it affects the fired glass or glaze is a key to control. |
| Glossary |
Unity Formula
The chemistry of ceramic glazes are normally expressed as formulas. A unity formula has been retotaled to make the numbers of flux oxides total one. |
| Glossary |
Frit
Frits are used in ceramic glazes for a wide range of reasons. They are man-made glass powders of controlled chemistry with many advantages over raw materials. |
| Glossary |
Chemical Analysis
In ceramics, raw material chemistry is expressed a chemical analyses. This is in contrast to fired glaze chemistries which are expressed as oxide formulas. |
| Articles |
Glaze Chemistry Basics - Formula, Analysis, Mole%, Unity
Part of changing your viewpoint of glazes, from a collection of materials to a collection of oxides, is learning what a formula and analysis are, how conversion between the two is done and how unity and LOI impact this. |
| Articles |
How desktop INSIGHT Deals With Unity, LOI and Formula Weight
INSIGHT enables you to enter material analyses as recipes. This is a first step to inserting them into the materials database. Imposing an LOI and understanding how to set unity, and its connection with formula weight are important concepts. |
| Oxides | KNaO - Potassium/Sodium Oxides |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://backup.digitalfire.com, All Rights Reserved
Privacy Policy
