| Monthly Tech-Tip | No tracking! No ads! |
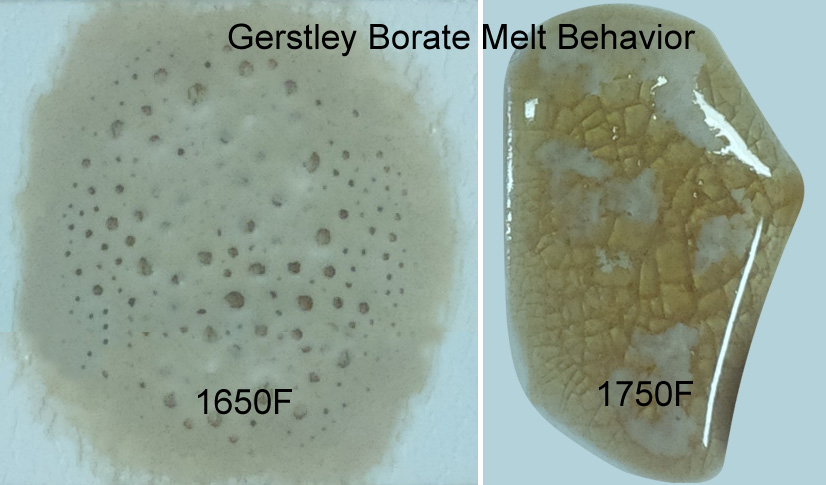
Why does Gerstley Borate melt in two stages? Because it is two minerals.
The ulexite in Gerstley Borate melts first, producing an opaque fired glass having the unmelted (and still gassing) particles of colemanite suspended in it. By 1750F the colemanite is almost melted also. Boron-containing frits, by contrast, soften slowly over a wide temperature range and gradually spread and melt. Not surprisingly they produce a more stable glaze (albeit often less interesting visually without additives e.g. titanium, rutile). These behavior contributes to phase changes in fired glazes that contribute to variegation.
Related Pictures
Gerstley Borate vs Frit 3134 melt fluidity comparison

This picture has its own page with more detail, click here to see it.
Here the melt fluidity of Gerstley Borate (GB) is being compared to Ferro Frit 3134 (using a GLFL test). Clearly, these are two very different materials. GB is a clay, Frit 3134 is a man made powdered glass. Notice the GB shrinks to about half its original size by 1600F and then suddenly by 1650 it has exploded out of the starting gate and crossed the finish line! The frit, conversely, slowly softens through the entire 1350-1650 range and then starts down the runway between 1650 and 1700F. While it is clear that frit 3134 is not a direct substitute for the Gerstley Borate (GB) it's more gradual melting make it a better source as a source of B2O3 (boron).
Videos
Links
| Oxides | B2O3 - Boric Oxide |
| Materials |
Colemanite
A natural source of boron that melts at a very low temperature. |
| Materials |
Ulexite
A natural source of boron, it melts at a very low temperature to a clear glass. |
| Materials |
Gerstley Borate
Gerstley Borate was a natural source of boron for ceramic glazes. It was plastic and melted clear at 1750F. Now we need to replace it. How? |
| Materials |
Gillespie Borate
A Gerstley Borate substitute that became available during the early 2000s and is still available in 2023. |
| Temperatures | Comparison of frit melts at 1750F (954-) |
Got a Question?
Buy me a coffee and we can talk

https://backup.digitalfire.com, All Rights Reserved
Privacy Policy